Origins of the Quantum Theory
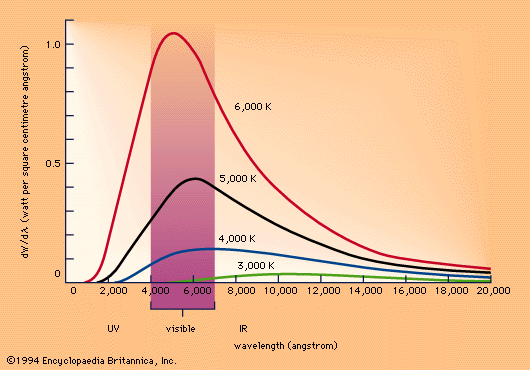

Heat a liquid or solid until it glows, and the light produced will have a continuous spectrum, the color changing from red to orange to white to blue as the temperature increases; this incandescent glow is the same for all elements. In 1900, Max Planck developed a formula to describe these phenomena; there was, however, no known theory to describe why it should fit the data. So Planck derived one. In the process, however, he had to reconsider the very nature of the conversion of light into heat: that there were discrete values at which it could occur and a smallest unit of exchange, which came to be known as the quantum. (The energy E of this exchange equals Planck's constant, or approximately 6.6 x 10^-34, times the frequency f of the light, or E=hf.)
Einstein in 1905 used Planck's idea of light quanta, or photons, to explain the photoelectric effect, wherein blue light shined on negatively charged metal would cause the charge to leak away, thus indicating that there might be a far broader significance to Planck's theory than simply explaining incandescence. Light, therefore, behaved like a particle in addition to a wave; quantum physics was born.
Maintained by adamse@technologist.com